Defects in FCC and BCC Iron
Christopher Bouthiette
MSE4984
This assignment studied both a surface defect in BCC Iron, and a vacancy in FCC and BCC Iron. Iron was chosen because of its crystalline structure and well known crystal structures. Iron is used in thousands of engineering applications most specifically as Steel. Steel being Iron with carbon constituents incorporated. Furthermore, Iron can be found in two main forms: BCC Iron and FCC Iron. BCC Iron is stiffer than FCC iron because of the crystal structure. Often Irons are alloyed with other elements to produce the desired mechanical characteristics.
Vacancies are point defects in crystalline materials. Vacancies are missing atoms from a lattice crystal structure. The number of vacancies and is often determined by the temperature of the material. The higher the temperature the higher the concentration of vacancies in a solid. Vacancies can often be responsible for creep and other failure mechanisms at higher temperatures. Vacancies also allow for impurity atoms to exist in a lattice, which can change material properties. Atomic simulations were done to simulate the vacancy formation energy.
Surface defects like cracks are formed when two surfaces separate. That is the bonds between the atoms in the two planes break. The formation of these surfaces is associated with a surface energy. The surface energy can then be related to the fracture toughness. Fracture toughness is of great interest to engineers and scientists because of the tendency of materials to exhibit brittle failure. Brittle failure is failure without yielding and is often catastrophic and fast. Atomic simulations were done to simulate the surface energy.
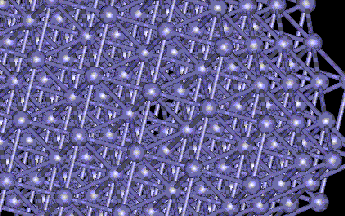
FCC Vacancy Formation

The vacancy can be seen at the small distortion of the atoms in the center of the picture. The defect energy of the vacancy is 5.858eV. The minimum energy of the block was -8587eV. The Maximum atomic displacement due to relaxation was 0.03911Angstroms.
BCC Vacancy Formation
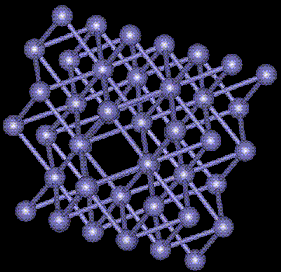
The vacancy can be seen at the distortion of the atoms in the center of the picture. The defect energy of the vacancy is 6.006eV. The minimum energy of the block was 6234eV. The Maximum atomic displacement due to relaxation was 0.08542Angstroms.
Surface energy of BCC Iron in the (210) plane
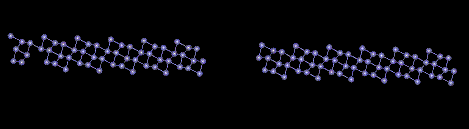
The surface defect seen above can be seen at the absence of the atoms in the center of the picture. The specific energy of the planar defect is 2.46J/m^2. The minimum energy of the block was 596eV. The Maximum atomic displacement due to relaxation was 0.2453 Angstroms.