Vacancies and Free Surfaces in Pure Iron
David C. Haeberle
Assignment 2
ESM 4984
Introduction
Iron-based alloys are some of the most widely used metals in the world. These alloys are usually used in structural applications. Iron alloys containing some carbon and manganese are called steels, those with excess carbon are called cast iron, and pure iron is called ingot iron. Pure iron, the material studied in this exercise, is not very useful because it is very weak. The addition of small amounts of carbon, however, increases its strength considerably. Pure iron can exist in two different atomic structures, body-centered cubic and face-centered cubic. The energy required to form vacancies in these two types of iron is calculated below, along with the specific surface energy for the [111] surface in the FCC structure. The calculation is completed using a computer analysis technique, which simulates the atomic structure. The vacancy formation energy, or energy of activation, is a necessary quantity in determining the number of vacancies in a given material, which may important for material processing, alloying, or material properties. The specific surface energy of a plane of materials is important for fracture evaluation, in fact, the ideal fracture strength of a material is based on surface energy.
BCC Vacancy
Lattice Parameter - 2.87 Angstroms
Cohesive Energy - 4.28 eV
Vacancy Formation Energy - 1.708157 eV
The atomic displacements observed are toward the vacancy in the atoms immediately next to the vacancy, but the next layer moves away from the vacancy. This phenomenon can be seen graphically in Figures 3 and 4 of the FCC vacancy (the same phenomenon occurs in FCC). Figure 1 shows the vacancy in the computer-generated BCC iron crystal.
Figure 1: BCC Iron Vacancy

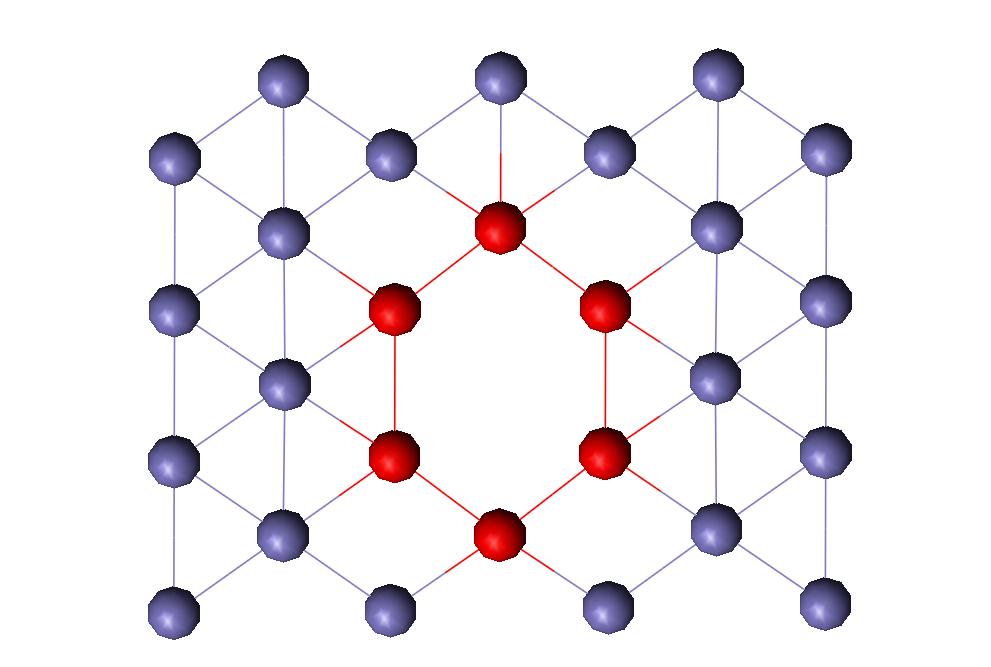
FCC Vacancy
Lattice Parameter - 3.515 Angstroms
Cohesive Energy - 4.196 eV
Vacancy Formation Energy - 1.665257 eV
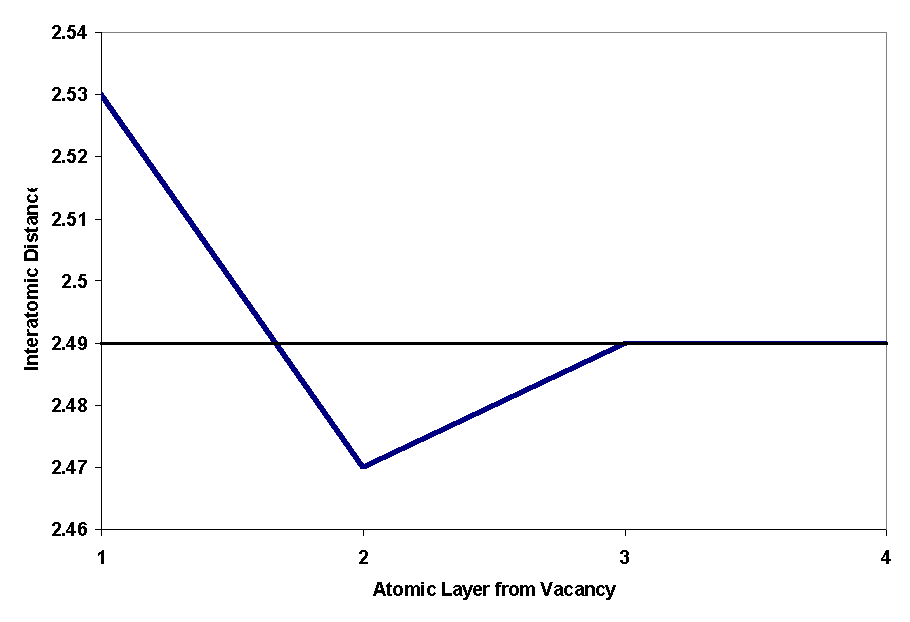
Figure 2 shows the vacancy in the computer-generated FCC iron crystal. Figure 3 shows the interatomic bond distances in a FCC plane as the atoms get further away from the vacancy. A graphical depiction of this phenomenon is shown in Figure 4.
Figure 2: FCC Iron Vacancy
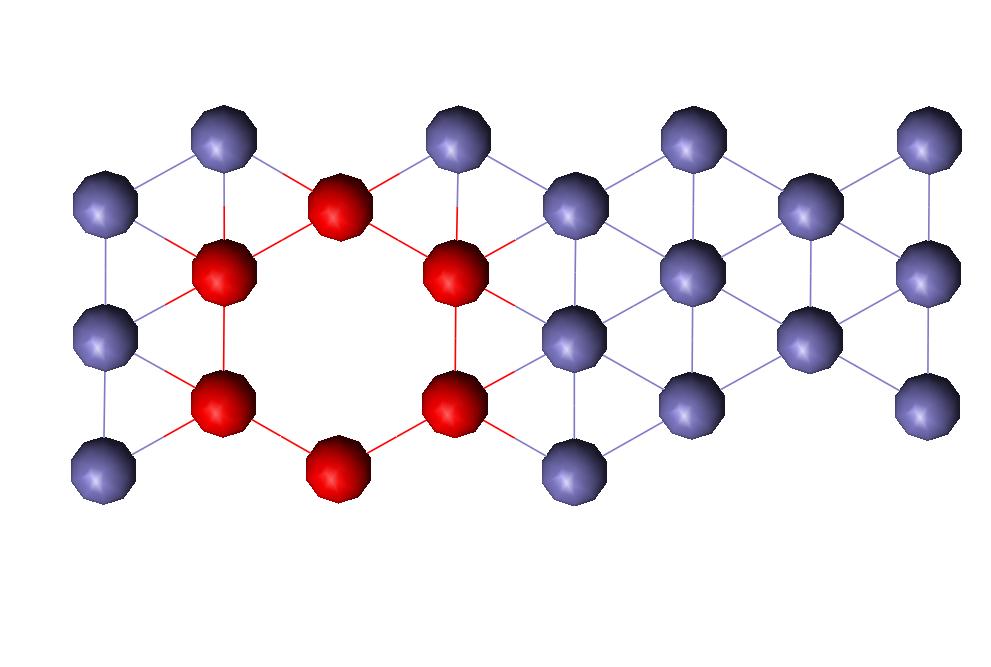
Figure 3: Interatomic Distance for FCC defect
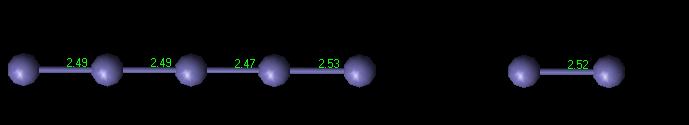
Figure 4: Graphical Representation of Atomic Distance
FCC Planar Defect
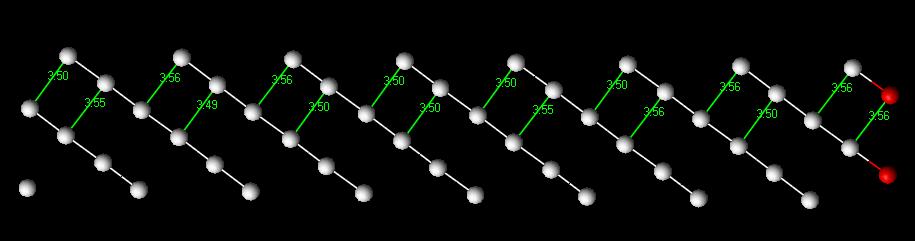
Computer generating a separation between atomic planes created the surface for the planar defect. The plane I created was the FCC [111] plane. This surface defect is shown in Figure 5. The interatomic distances do change as they get further away from the defect, but it appears that most of the distances are above the normal for two layers, then below for two layers until it converges upon the normal interatomic distance (shown in Fig. 6). It was expected that the distances between planes would alternate between high and low numbers as seen in current literature and with the vacancy simulations.
Specific Surface Energy - 1.816 J/m2
Figure 5: FCC Surface Defect
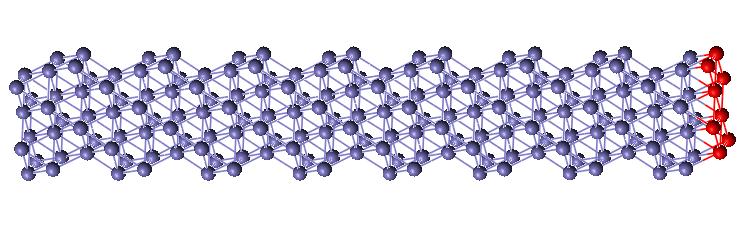
Figure 6: FCC Surface Defect Interatomic Distance